Can I Get Social Security Disability Benefits for Hepatitis or Other Chronic Liver Diseases?
- How Does the Social Security Administration Decide if I Qualify for Disability Benefits for Chronic Liver Disease?
- About Chronic Liver Disease and Disability
- Winning Social Security Disability Benefits for Liver Disease by Meeting a Listing
- Residual Functional Capacity Assessment for Chronic Liver Disease
- Getting Your Doctor’s Medical Opinion About What You Can Still Do
Winning Social Security Disability Benefits for Liver Disease by Meeting a Listing
To determine whether you are disabled at Step 3 of the Sequential Evaluation Process, the Social Security Administration will consider whether your liver disease is severe enough to meet or equal the chronic liver disease listing. The Social Security Administration has developed rules called Listing of Impairments for most common impairments. The listing for a particular impairment describes a degree of severity that Social Security Administration presumes would prevent a person from performing substantial work. If your liver disease is severe enough to meet or equal the listing, you will be considered disabled.
The listing for chronic liver disease is 5.05. It has 7 parts, A through G. You will be disabled if you satisfy any part. Regardless of cause, you must establish that you have chronic liver disease to satisfy any part of the listing. The Social Security Administration defines chronic as persisting more than six months.
There are many types and causes of chronic liver disease. Instead of picking out criteria for a huge number of possible liver disorders, the Social Security Administration has created the listing to cover the fundamental complications that could produce functional limitations or death regardless of specific cause.
Meeting Social Security Administration Listing 5.05A for Liver Disease
You will meet listing 5.05A if you have chronic liver disease with:
- Hemorrhaging from esophageal, gastric, or ectopic varices or from portal hypertensive gastropathy;
- Demonstrated by endoscopy, X-ray, or other appropriate medically acceptable imaging;
- Resulting in hemodynamic instability; and
- Requiring hospitalization for transfusion of at least 2 units of blood.
If you meet this listing, you will be considered under a disability for 1 year following your last documented transfusion. Thereafter, you will be evaluated for residual impairments.
Varices
Varices are dilated areas in blood vessels (areas with enlarged diameters). Sometimes varices rupture and bleed. Part A of the listing recognizes that severe liver disease can cause varices and their potential complication of life-threatening bleeding in the esophagus and stomach (gastric varices). The broad term “ectopic varices” also recognizes other less likely locations such as the gallbladder.
Esophageal Varices
The liver is connected to the portal venous system that receives blood from the intestines. The portal vein provides about 75% of the liver’s blood flow (see Figure 2 below).
The esophageal veins drain into the portal system. Cirrhosis of the liver results in increased pressure inside the portal system, known as portal hypertension. Abnormal pressures are transmitted back to the esophageal veins, which are delicate and close to the inner surface of the esophagus. Dilated areas (esophageal varices) can develop in these veins.
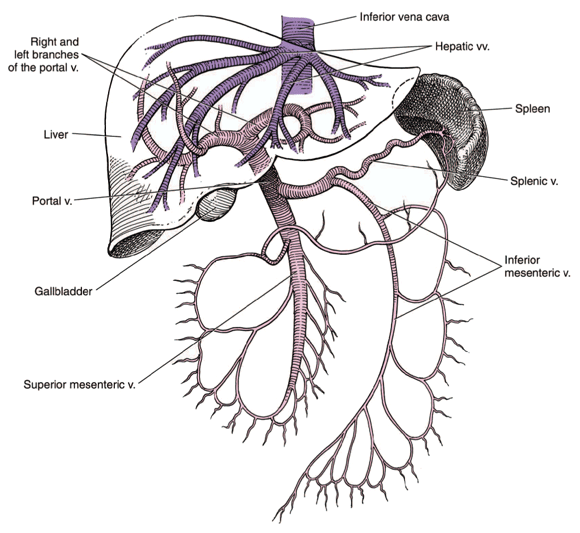
Figure 2: The hepatic portal vein system.
When bleeding does occur, it can be minor, or life-threatening. By far, most of the bleeding esophageal varices seen by the Social Security Administration are in alcoholics. However, since other types of cirrhosis—such as that caused by hepatitis C infection—also can result in varices, bleeding should not result in a presumption of alcoholism.
Fifty to 75% of people who have had bleeding varices will experience re-bleeding within 1 to 2 years of a bleeding episode. Bleeding varices are usually treated with fiberoptic endoscopy. MRIs and CT scans to image varicosed veins in the esophagus and intra-abdominally are also useful tools that may be part of the diagnostic work-up. However, endoscopy is most important for diagnosis of esophageal and gastric varices.
Esophageal varices are frequently treated initially with sclerotherapy, in which a scarring solution is injected into varices as an endoscopic procedure. However, if sclerotherapy does not prevent bleeding, more definitive treatment may be needed, and that is shunt surgery.
Portal Hypertensive Gastropathys
Portal hypertensive gastropathy (PHG) refers to changes in the stomach lining of patients with portal hypertension. The most common cause of PHG is cirrhosis of the liver. In patients with PHG, the the stomach mucosa has a snake skin appearance that is radically different from the mucosa’s usual smooth look. Patients with portal hypertensive gastropathy may experience bleeding from the stomach. When there are red spots throughout the stomach, the term diffuse gastric vascular ectasia is used.
It has been estimated that nearly two-thirds of patients with cirrhosis will eventually develop PHG. This disorder is frequently associated with varices in the esophagus or stomach, but they are not necessary for a diagnosis of PHG. Furthermore, PHG can cause life-threatening bleeding without varices in the esophagus or elsewhere.
PHG is usually diagnosed by endoscopy.
PHG is most often found in the more proximal or upper parts of the stomach closer to the esophagus. It accounts for about 10% of all causes of gastrointestional bleeding and about 25% of cases of bleeding in individuals with portal hypertension.
Beta-blocker drugs have been useful in prevention of chronic blood loss. Iron supplementation is required. A portocaval shunt (requiring surgery) or a transjugular intrahepatic portal-systemic shunt (TIPS) may decrease the risk of bleeding by lowering venous pressures and is used when medications fail. Argon photocoagulation therapy has also been used, and some experiments have been done with carbon dioxide cryotherapy to stop acute bleeding.
Gastric Vascular Antral Ectasia
Gastric vascular antral cctasia (GAVE, GVE) is not mentioned by the listing, but should be considered as of equivalent severity to portal hypertensive gastropathy (PHG). GAVE is associated with bleeding from abnormal vessels in the distal (lower) stomach. It may not have the snakeskin pattern in the mucosa. As with PHG, stabilization of iron stores from blood loss is needed. Estrogen-progesterone combination drug therapy has been of some use when thermoablative argon laser photocoagulation has been ineffective.
Unlike PHG, GAVE may be more related to liver failure than to portal hypertension caused by liver failure, because liver transplantation leads to a cure. If bleeding continues despite thermoablative therapy and hormonal treatment, then surgical resection of the distal stomach (antrum) has been done. Unlike PHG, a shunt (TIPS) is not a treatment for GAVE, because it does not significantly reduce the risk of bleeding and is associated with a much higher risk of hepatic encephalopathy.
Fortunately, GAVE is not as common as PHG.
Bleeding and Transfusion Requirements
GI bleeding can be vomiting blood (hematemesis), tarry stools (melena), or bloody stools (hematochezia). The manner of blood loss is not important. Hospitalization to provide supportive care for life-threatening bleeding must be documented. You must require at least 2 units of blood at least 30 days apart, at least three times in a consecutive 6-month period. You must require hospitalization for each episode.
Signs of Hemodynamic Instability
To further substantiate that blood loss is severe and resulting from bleeding, rather than one of the many causes of anemia, there must be evidence of hemodynamic instability, that is, severe physiological and measurable changes resulting from inadequate perfusion of tissues.
These signs are:
- Pale skin (pallor);
- Profuse perspiration (diaphoresis);
- Rapid pulse (tachycardia);
- Low blood pressure (hypotension);
- Falling blood from the act of standing up from lying down (postural hypotension); and
- Fainting (syncope).
The Social Security Administration should not require all of these signs of hemodynamic instability to be documented, because the reality is that hospital records are not always of the quality they should be. However, in the total context of a patient’s condition, the data should indicate such instability.
Meeting Social Security Administration Listing 5.05B for Liver Disease
You will meet listing 5.05B if you have chronic liver disease with:
- Ascites or hydrothorax not attributable to other causes,
- Despite continuing treatment as prescribed,
- Present on at least two evaluations at least 60 days apart within a consecutive 6-month period.
Each evaluation must be documented by:
1. Paracentesis or thoracentesis; or
2. Appropriate medically acceptable imaging or physical examination and one of the following:
a. Serum albumin of 3.0 g/dL or less; or
b. International Normalized Ratio (INR) of at least 1.5.
Ascites
Ascites means an abnormal accumulation of fluid in the abdomen. If of hepatic origin, ascites is a sign of very serious liver disease. In advanced liver disease, the liver cannot produce a normal amount of albumin, and this can result in a disruption of fluid balance between the body’s internal compartments. If there is enough ascites, a doctor can palpate it in a patient’s abdomen on a physical examination. But this method of detection is not reliable, especially in obese claimants.
In a non-obese person, ascites is often easily demonstrable based on a patient’s history and physical examination when there is severe cirrhosis and the abdomen is large and bulging. It takes about 1.5 liters (1500 ml) of ascitic fluid to be detected on physical examination.
Paracentesis is a procedure by which a physician inserts a needle into the abdomen to remove some of the ascitic fluid. The Social Security Administration never orders this test because of its invasive nature. The clinical basis for paracentesis is to discover the cause of ascites, not prove its presence. If available, however, abdominal fluid obtained by paracentesis is irrefutable proof of ascites and its chemical content may establish that liver disease is the cause of ascites.
Ascites can be caused by cirrhotic liver disease, a condition that in the U.S. most often results from alcohol abuse and hepatitis C infection.
In addition to liver disease, ascites can also be associated with a number of other disorders, such as cancer (breast, colon, lung, ovarian, pancreatic), constrictive pericarditis, congestive heart failure, kidney disease (nephrotic syndrome) and infection (e.g., tuberculosis).
Hydrothorax
Hydrothorax refers to accumulation of fluid in the chest. When due to liver disease, hydrothorax is thought to arise in association with ascites, and is defined as a pleural effusion of more than 500 ml. In hydrothorax, some of the ascitic fluid moves across the peritoneal membrane lining the abdominal cavity through defects in the right side of the diaphragm, some which might even be microscopic.
While patients can tolerate 5 to 10 liters of fluid in their abdomens, they are far less tolerant of hydrothorax with shortness of breath, cough, and even de-oxygenation (hypoxemia) possible with 1 to 2 liters of fluid accumulation.
In the first attempt to control hepatic hydrothorax, sodium restricton and diuretics may be given. A second option if that fails is a transjugular intrahepatic portosystemic shunt (TIPS). A transjugular intrahepatic portosystemic shunt (TIPS) is a small, metal tube commonly called a stent that is placed in veins in the middle of the liver to permit blood flow to bypass the liver.
Thoracentesis is a procedure by which fluid can be removed from the chest. Basically, a tube is inserted through the chest wall and fluid drained out under sterile conditions. Thoracentesis may provide only temporary relief until the pleural effusion is large again. The rate of recurrence depends on the patient; it could be only a couple of weeks. In chronic hepatic hydrothorax, a more permanent solution would be required.
On physical examination for pleural effusions like a hydrothorax, there is dullness to percussion with finger-tapping and decreased or absent breath sounds (to a stethoscope) over fluid-filled areas, the positioning of which can change with changes in the patient’s body position. In obese individuals, such examination becomes much less reliable.
There are many causes of hydrothorax, other than liver disease, such as pneumonia, cancer, and autoimmune diseases.
Medically Acceptable Imaging
Several imaging techniques can demonstrate both ascites and hydrothorax, including X-rays, ultrasound, and magnetic resonance imaging. Any of these would be acceptable, and the Social Security Administration should pay for these tests if needed to adjudicate your claim.
Blood Tests
If imaging or physical exam, rather than paracentesis or thoracentesis, are the means of diagnosis of continuing abnormality, then either a low serum albumin (hypoalbuminemia) or an abnormal International Normalized Ratio (INR) must be demonstrated. These are simple blood tests and the Social Security Administration could purchase either if needed to adjudicate your claim.
Part 2.a requires hypoalbuminemia demonstrated by an abnormal serum level of 3.0 gm/dl or less (normal = 3.5 – 5.5 gm/dl). With ascites and advanced liver disease, it is common for serum albumin to decrease. Not only is the liver less able to produce albumin, but much is lost into the ascitic fluid.
Part 2.b requires an abnormal INR of 1.5 or more. Since the liver makes coagulation factor proteins like prothrombin, advanced liver disease can result in coagulation abnormalities. Therefore, the degree of coagulation abnormality can be used as a guide to the severity of liver disease. An INR value of 1.5 indicates a mild degree of coagulation abnormality, so the Social Security Administration is simply trying to establish the presence of a severe liver disorder—not to establish a severe bleeding disorder.
Meeting Social Security Administration Listing 5.05C for Liver Disease
You will meet listing 5.05C if you have chronic liver disease with spontaneous bacterial peritonitis with peritoneal fluid containing an absolute neutrophil count of at least 250 cells/mm.
Spontaneous bacterial infection of abdominal ascitic fluid is a risk factor in chronic liver disease. “Spontaneous” means that there is no evident cause of infection, such as surgical contamination or a ruptured appendix. As infection gets worse, neutrophilic white blood cells are attracted to the ascitic fluid as part of a normal immune response. In the absence of infection, they would not be present in any significant number.
“Absolute” means there has to be a reported number of neutrophils—a percentage of neutrophils in a total white cell count is not acceptable as equivalent. Only one documentation of infection is required. There are many other causes of ascites with infection other than liver disease, and they would not be applicable to this liver disease listing.
Meeting Social Security Administration Listing 5.05D for Liver Disease
You will meet listing 5.05D if you have chronic liver disease with hepatorenal syndrome, with one of the following:
1. Serum creatinine elevation of at least 2 mg/dL; or
2. Oliguria with 24-hour urine output less than 500 mL; or
3. Sodium retention with urine sodium less than 10 mEq per liter.
Hepatorenal syndrome is defined as functional renal failure associated with chronic liver disease in the absence of underlying kidney pathology. Hepatorenal syndrome is a functional kidney failure caused by advanced liver disease, usually cirrhosis and ascites. “Functional” means the kidneys are not anatomically damaged—reversal of liver disease such as with a liver transplant—will result in a return of normal kidney function. In fact, you could transplant such kidneys into someone without liver disease and the organs would function normally.
Hepatorenal syndrome is associated with elevation of serum creatinine, decreased urine output (oliguria), and retention of abnormal amounts of sodium. Decreased renal blood flow and a number of other abnormalities have been postulated as a cause of this disorder, but the pathophysiology is not clear.
Any one of the part D criteria measured one time is sufficient. These abnormalities do not in themselves establish hepatorenal syndrome. Evidence for advanced liver disease causing these abonormal test results is needed.
Meeting Social Security Administration Listing 5.05E for Liver Disease
You will meet listing 5.05E if you have chronic liver disease with hepatopulmonary syndrome, with:
1. Arterial oxygenation (PaO2) on room air of:
a. 60 mm Hg or less, at test sites less than 3000 feet above sea level, or
b. 55 mm Hg or less, at test sites from 3000 to 6000 feet, or
c. 50 mm Hg or less, at test sites above 6000 feet; or
2. Documentation of intrapulmonary arteriovenous shunting by contrast-enhanced echocardiography or macroaggregated albumin lung perfusion scan.
Hepatopulmonary syndrome (HPS) refers to three things occurring together: systemic deoxygenation of arterial blood (hypoxemia), advanced liver disease, and abnormal vascular dilation (blood vessel widening ) in the lungs.
HPS patients have shortness of breath (dyspnea), increased deoxygenation of blood on standing (orthodeoxia), improved shortness of breath lying flat (platypnea), and signs of hypoxemia like clubbing of the fingers and cyanosis (bluish discoloration of skin). Supplemental oxygen is given to treat hypoxemia. There is no good treatment for HPS, though liver transplantation can reverse it. Survival is usually less than 2 years.
The test preferred for diagnosis is contrast echocardiography. Normally, intravenous saline containing microbubbles created by agitation are trapped within the lung. In HPS, the echocardiogram can detect bubbles transiting the lung and reaching the heart within 7 beats.
Parts E.1a through c can be satisfied by hypoxemia of 60, 55, and 50 mm Hg, respectively, for testing facilities of different altitudes at 3000, 3000-6000, and above 6000 feet. These numbers are not valid unless performed on room air. They might be falsely elevated with supplemental oxygen. No more than one valid test is necessary to satisfy this part of the listing.
Arterial blood gas studies (ABGS) should be done in the standing, or at least sitting, position, as these may be lower than when you are lying flat. However, if your tests results while lying satisfy the listing it would be silly to require measurements in a more upright position as they would certainly be worse.
Part E.2 can be satisfied by either contrast echocardiography or technetium-99 lung scanning.
Meeting Social Security Administration Listing 5.05F for Liver Disease
You will meet listing 5.05F if you have chronic liver disease with hepatic encephalopathy with 1 and either 2 or 3:
1. Documentation of abnormal behavior, cognitive dysfunction, changes in mental status, or altered state of consciousness (for example, confusion, delirium, stupor, or coma), present on at least two evaluations at least 60 days apart within a consecutive 6-month period; and
2. History of transjugular intrahepatic portosystemic shunt (TIPS) or any surgical portosystemic shunt; or
3. One of the following occurring on at least two evaluations at least 60 days apart within the same consecutive 6-month period as in F1:
a. Asterixis or other fluctuating physical neurological abnormalities; or
b. Electroencephalogram (EEG) demonstrating triphasic slow wave activity; or
c. Serum albumin of 3.0 g/dL or less; or
d. International Normalized Ratio (INR) of 1.5 or greater.
Hepatic encephalopathy is a potentially reversible organic brain dysfunction, particularly associated with cirrhosis and portal hypertension. Ammonia is a waste product of protein metabolism that has a toxic effect on brain cells. Ammonia is formed by the action of bacteria in the colon on urea and protein. It is then absorbed into the portal venous system, as are other toxins. The portal vein carries this blood to the liver, where a healthy liver normally extracts most ammonia and other toxins before they can reach the brain. But a diseased liver cannot clear the toxins from the blood. Some ammonia is also produced in the kidneys. Blood ammonia levels generally correlate with the severity of the encephalopathy. Normal ammonia levels are about 11 to 35 micromoles/liter.
GRADING OF HEPATIC ENCEPHALOPATHY
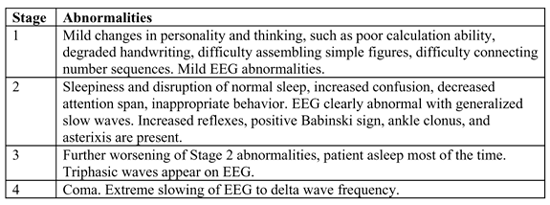
Part F.1 describes mental abnormalities that can be caused by hepatic encephalopathy. The listing does not specify that these findings must be provided by a psychiatrist. If symptoms are severe and fit the total historical and clinical context for the patient, there is no reason that the observations of the attending non-psychiatrist couldn’t suffice. Unclear cases should be evaluated by a psychiatrist to obtain a full, detailed, mental status examination. In cases of severe liver disease, the Social Security Administration should never assume intact mental status simply because the claimant does not allege mental status problems, or because medical records do not address the question of encephalopathy. This part of the listing requires more refined medical judgment by the adjudicator than the other parts of the listing. However, F.1 must be satisfied. There must be at least two examinations at least 60 days apart, over a consecutive period of 6 months.
You will meet the listing if you have had a transjugular intrahepatic portosystem shunt (TIPS) and satisfy the requirements in F.1. There is no time limit on qualifying by having a TIPS, the need for which implies a poor prognosis.
If a TIPS has not been inserted, then the criteria in F.3 have to be satisfied at least twice, 60 or more days apart, within a consecutive 6-month period.
F.3.a refers to asterixis (a flapping tremor, particularly of the hand) or other fluctuating neurological abnormality which presumably could be a variety of tremors, movement disorders, and so forth.
The EEG abnormalities in F.3.b correspond to Stage 3 in the above table. The EEG report should specifically note the presence of the triphasic abnormality. This finding would be in a pre-coma, hospitalized patient who is asleep most of the time.
F.3.c and F.3.d for serum albumin and INR of 3.0 g/dL and 1.5, respectively, can easily be obtained by simple blood tests.
Any combination of F.3.a through d on the two testing times should satisfy the listing as long as the testing time parameters are correct.
Meeting Social Security Administration Listing 5.05G for Liver Disease
You will meet listing 5.05G if you have end stage liver disease with SSA CLD scores of 22 or greater. The SSA CLD score is calculated using formulas that include three laboratory values: (1) serum total bilirubin (mg/dL), (2) serum creatinine(mg/dL), and (3) International Normalized Ratio (INR). All of the required laboratory values must have been obtained within 30 days of each other.
Part G is an attempt by the Social Security Administration to objectively and consistently evaluate hepatic biochemical abnormalities that identify cases of end stage liver disease (ESLD). In end stage liver disease the liver has only minimal function. The only treatment is a liver transplant.
The Social Security Administration provides detailed formulas for how the score is calculated. The formulas are based on accepted data in the medical literature. Specifically, they are based on the Model for End Stage Liver Disease (MELD) calculation. The MELD is a numerical scale developed for the United Network for Organ Sharing (UNOS) that is used for liver allocation within the Organ Procurement and Transplantation Network. The MELD score estimates an individual’s risk of dying while waiting for a liver transplant.
Listing 5.05G requires two SSA CLD scores. The laboratory values for the second SSA CLD score calculation must have been obtained at least 60 days after the latest laboratory value for the first SSA CLD score and within a 6-month period. The Social Security Administration will consider the date of each SSA CLD score to be the date of the first laboratory value used for its calculation.
If you have the two SSA CLD scores required by 5.05G, the Social Security Administration will consider that your impairment meets the criteria of the listing from at least the date of the first SSA CLD score.
Continue to Residual Functional Capacity Assessment for Chronic Liver Disease.
Go back to About Chronic Liver Disease and Disability.
